The rate constant for a particular zero-order reaction is 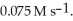 If the initial concentration of reactant is
If the initial concentration of reactant is 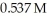 it takes ________ s for the concentration to decrease to
it takes ________ s for the concentration to decrease to 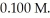
A) 5.8
B) -5.8
C) -0.047
D) 7.2
E) 0.040
Correct Answer:
Verified
Q81: The overall reaction below is _ and
Q82: The combustion of ethylene proceeds by the
Q83: The rate of disappearance of HBr in
Q84: The reaction A → B is first
Q85: The isomerization of methylisonitrile to acetonitrile CH3NC
Q87: Nitrogen dioxide decomposes to nitric oxide and
Q88: At elevated temperatures,dinitrogen pentoxide decomposes to nitrogen
Q89: The rate of disappearance of HBr in
Q90: What value is represented by the y-intercept
Q91: What is the rate constant (
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents