The elementary reaction 2NO2 (g) → 2NO (g) + O2 (g)
Is second order in 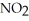 and the rate constant at 660 K is 5.23
and the rate constant at 660 K is 5.23 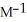
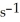 .The reaction half-life at this temperature when
.The reaction half-life at this temperature when 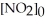 = 0.45 M is ________ s.
= 0.45 M is ________ s.
A) 2.4
B) 7.6
C) 0.19
D) 0.13
E) 0.42
Correct Answer:
Verified
Q97: The following reaction is second order in
Q98: A compound decomposes by a first-order process.If
Q99: Of the units below,_ are appropriate for
Q100: The rate law for a reaction is
Q101: Define Beer's Law.
Q103: The rate constant for a second-order reaction
Q104: At elevated temperatures,nitrogen dioxide decomposes to nitrogen
Q105: The rate-determining step is the _ elementary
Q106: The isomerization of methylisonitrile to acetonitrile CH3NC
Q107: A first-order reaction has a rate constant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents