Multiple Choice
A first-order reaction has a rate constant of 0.33 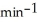 .It takes ________ min for the reactant concentration to decrease from 0.13 M to 0.066 M.
.It takes ________ min for the reactant concentration to decrease from 0.13 M to 0.066 M.
A) 0.085
B) 0.13
C) 0.89
D) 2.4
E) 2.1
Correct Answer:
Verified
Related Questions
Q102: The elementary reaction 2NO2 (g)→ 2NO (g)+
Q103: The rate constant for a second-order reaction
Q104: At elevated temperatures,nitrogen dioxide decomposes to nitrogen
Q105: The rate-determining step is the _ elementary
Q106: The isomerization of methylisonitrile to acetonitrile CH3NC
Q108: The reaction 2NOBr (g)→ 2 NO (g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents