Multiple Choice
The 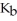 for
for 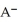 is
is 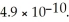 What is the pOH of a 0.0727 M aqueous NaA solution at 25.0 °C?
What is the pOH of a 0.0727 M aqueous NaA solution at 25.0 °C?
A) 9.33
B) 5.22
C) 8.78
D) 1.14
E) 10.00
Correct Answer:
Verified
Related Questions
Q129: The pH of a 0.25 M aqueous
Q130: Of the following,which is the strongest acid?
A)HIO4
B)HIO3
C)HIO2
D)HIO
E)The
Q131: A Lewis acid is an electron-pair acceptor,and
Q132: The Q133: The pOH of a 0.25 M aqueous Q134: What is the pH of a 0.40 Q135: What is the pH of 0.626 M Q136: Of the following,which is the weakest acid? Q137: The Q138: In the reaction![]()
A)HPO3-
B)H3PO4
C)H2PO4-
D)HPO4-
E)The![]()
BF3 + F- → BF4-
BF3
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents