All quantum states forming a shell have the same
A) principal quantum number n.
B) orbital quantum number 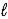 .
.
C) orbital magnetic quantum number 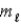 .
.
D) n, 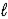 and
and 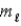
E) n and 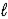 only.
only.
Correct Answer:
Verified
Q42: What is the difference in frequency for
Q43: A hydrogen atom emits a photon of
Q44: A headwaiter at a restaurant decides to
Q48: What is the difference in wavelength for
Q49: Aline says that the magnetic moment of
Q51: All quantum states forming a sub-shell have
Q52: Suppose a beam of electrons is incident
Q52: Zeke says that the magnitude of the
Q53: Quantum physics agrees with the classical physics
Q56: In an atom that has an electron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents