A laser (with wavelength of 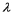 ) excites the electron in a hydrogen atom from its ground state to its first excited state.What laser wavelength is required to raise the electron from the first excited state to its next lowest excited state?
) excites the electron in a hydrogen atom from its ground state to its first excited state.What laser wavelength is required to raise the electron from the first excited state to its next lowest excited state?
A) 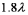
B) 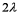
C) 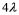
D) 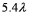
E) 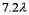
Correct Answer:
Verified
Q33: A group of hydrogen-like atoms (atoms with
Q34: A hydrogen atom is in a quantum
Q35: A hydrogen atom is in a quantum
Q36: A hydrogen atom relaxes to a lower
Q37: He+ is a helium atom with one
Q39: The angular frequency of rotation of electron
Q40: Find the frequency of light emitted by
Q41: What is the energy of the orbiting
Q42: If the energy of the orbiting electron
Q43: A photon of wavelength 1.17
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents