Hydrogen sulfide, H2S, burns in the presence of oxygen, 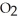 , to produce water,
, to produce water, 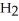 O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2
O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2 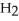 S + 3
S + 3 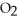 → 2
→ 2 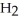 O + 2
O + 2 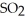
A) Sulfur is reduced.
B) Sulfur is oxidized
C) Sulfur is both oxidized and reduced.
D) Sulfur is neither oxidized nor reduced.
Correct Answer:
Verified
Q22: What correlation might you expect between an
Q26: The general chemical equation for photosynthesis is
Q28: Consider the following molecules. What is the
Q29: Chemical equations need to be balanced not
Q30: What might the relationship be between an
Q32: Which element is closer to the upper
Q34: What element is oxidized in the following
Q35: What might the relationship be between an
Q35: The general chemical equation for photosynthesis is
Q120: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents