The general chemical equation for photosynthesis is shown below. Through this reaction is the carbon oxidized or reduced? How can you tell? 6 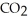 + 6
+ 6 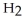 O →
O → 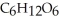 + 6
+ 6 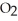
A) Oxidized, since carbon is in the +4 oxidation state in 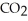 but in the +6 oxidation state in the product,
but in the +6 oxidation state in the product, 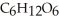 , glucose.
, glucose.
B) Reduced, since in carbon dioxide there are two oxygen atoms for every one carbon but within the product, 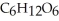 , (glucose) , there is only one oxygen for every one carbon.
, (glucose) , there is only one oxygen for every one carbon.
C) Neither, since carbon does not change its oxidation state and is neither oxidized nor reduced.
D) Both, since the carbon atoms within the glucose molecules display two different charge states.
Correct Answer:
Verified
Q22: What correlation might you expect between an
Q30: What might the relationship be between an
Q30: Hydrogen sulfide, H2S, burns in the presence
Q32: Which element is closer to the upper
Q34: What element is oxidized in the following
Q37: What element is behaves as the oxidizing
Q38: Iron atoms, Fe, are better reducing agents
Q40: For each of the following unbalanced equations,
Q101: How does an atom's electronegativity relate to
Q133: Clorox is a laundry bleaching agent used
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents