The figure shows a pV diagram for 2.9 g of ideal oxygen gas O2 in a sealed container. The temperature of state 1 is 76° C, the atomic mass of the oxygen atom is 16 g/mol, and R = 8.31 J/mol ∙ K. What are the temperatures T3 and T4? 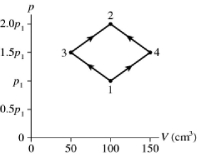
A) -11° C and 510° C
B) 57° C and 170° C
C) 260° C and 790° C
D) 38° C and 110° C
Correct Answer:
Verified
Q67: If we add 700 J of heat
Q79: A sample of ideal monatomic gas is
Q82: A gas expands from an initial volume
Q90: A gas expands from an initial volume
Q211: The figure shows a pV diagram
Q213: The figure shows a pV diagram for
Q215: A sealed 87-
Q216: The figure shows a pV diagram for
Q217: The temperature of an ideal gas
Q218: The figure shows a pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents