If 5.00 g Br2 and 1.10 g NH3 react according to the equation below,what is the maximum mass of ammonium bromide produced?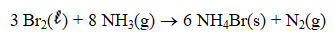
A) 3.06 g
B) 6.13 g
C) 12.9 g
D) 4.74 g
E) 8.43 g
Correct Answer:
Verified
Q8: How many moles of sodium bromide
Q9: If 48.8 g of O2 is mixed
Q10: The commercial production of phosphoric acid,H3PO4,can
Q11: Pure copper may be produced by
Q12: How many grams of dioxygen are required
Q14: How many moles of Mg3P2(s)can be
Q15: The compound P4S3 is used in
Q16: Calculate the number of moles of O2
Q17: If the complete combustion of an
Q18: Potassium hydrogen carbonate decomposes with heat
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents