Hydrazine,N2H4,is a liquid used as a rocket fuel.It reacts with oxygen to yield nitrogen gas and water.
N2H4( 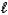 ) + O2(g) N2(g) + 2 H2O(
) + O2(g) N2(g) + 2 H2O( 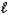 )
)
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
A) -19.4 kJ/mol
B) -25.6 kJ/mol
C) -126 kJ/mol
D) -622 kJ/mol
E) -820.kJ/mol
Correct Answer:
Verified
Q44: Determine
Q45: A bomb calorimeter has a heat capacity
Q46: Determine the standard enthalpy of formation of
Q47: Iron oxide reacts with aluminum in
Q48: How much heat is liberated at
Q50: Combustion of 7.21 g of liquid
Q51: Determine the enthalpy change for the
Q52: When 10.0 g KOH is dissolved
Q53: A chemical reaction in a bomb
Q54: When 50.0 mL of 1.30 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents