Determine the standard enthalpy of formation of Fe2O3(s) given the thermochemical equations below.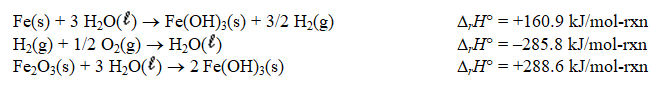
A) -252.6 kJ/mol-rxn
B) +163.7 kJ/mol-rxn
C) -824.2 kJ/mol-rxn
D) +33.2 kJ/mol-rxn
E) + 890.6 kJ/mol-rxn
Correct Answer:
Verified
Q41: Which of the following has a standard
Q42: The overall chemical equation resulting from
Q43: Commercial cold packs consist of solid
Q44: Determine
Q45: A bomb calorimeter has a heat capacity
Q47: Iron oxide reacts with aluminum in
Q48: How much heat is liberated at
Q49: Hydrazine,N2H4,is a liquid used as a
Q50: Combustion of 7.21 g of liquid
Q51: Determine the enthalpy change for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents