Hydrogen peroxide decomposes into water and oxygen in a first-order process.H2O2(aq) H2O( 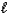 ) + 1/2 O2(g)
) + 1/2 O2(g)
At 20.0 C,the half-life for the reaction is 3.92 104 seconds.If the initial concentration of hydrogen peroxide is 0.52 M,what is the concentration after 7.00 days?
A) 1.2 10-5 M
B) 0.034 M
C) 0.074 M
D) 0.22 M
E) 0.52 M
Correct Answer:
Verified
Q35: In general,as temperature increases,the rate of a
Q48: The decomposition of phosphine,PH3,follows first-order kinetics.
4
Q49: A second-order reaction starts with an
Q50: For the zero-order reaction below,a graph
Q52: Calculate the activation energy,Ea,for
N2O5(g)
Q54: For the second-order reaction below,the initial
Q55: The rate constant for a first-order
Q56: The Arrhenius equation, Q57: According to collision theory,which condition(s)must be met Q58: A first-order reaction is 40.0% complete![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents