The Arrhenius equation, 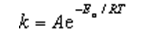 ,relates the rate constant of reaction and temperature.A plot of ____ versus 1/T will yield a straight line with a slope of -Ea/R.
,relates the rate constant of reaction and temperature.A plot of ____ versus 1/T will yield a straight line with a slope of -Ea/R.
A) k2/k1
B) -Ea
C) ln(k)
D) 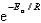
E) 1/RT
Correct Answer:
Verified
Q35: In general,as temperature increases,the rate of a
Q52: Calculate the activation energy,Ea,for
N2O5(g)
Q53: Hydrogen peroxide decomposes into water and
Q54: For the second-order reaction below,the initial
Q55: The rate constant for a first-order
Q57: According to collision theory,which condition(s)must be met
Q58: A first-order reaction is 40.0% complete
Q59: The rate constant for a reaction at
Q60: What is the half-life of a
Q61: A possible mechanism for the gas phase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents