A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation: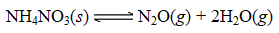
At equilibrium the total pressure in the container was found to be 2.81 atm at a temperature of 500.°C.Calculate Kp.
A) 1.75
B) 0.877
C) 3.29
D) 88.8
E) 0.822
Correct Answer:
Verified
Q48: A 2.50-mol sample of HI is
Q49: Nitrosyl bromide decomposes according to the
Q50: For the reaction given below,2.00 moles of
Q51: Sulfuryl chloride decomposes to sulfur dioxide and
Q52: In an experiment,0.42 mol H2 and 0.42
Q54: At 25
Q55: A mixture of nitrogen and hydrogen was
Q56: For the equilibrium N2O4(g) 
Q57: At 800 K,the equilibrium constant,Kp,for the
Q58: At 700 K,Kp for the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents