At 800 K,the equilibrium constant,Kp,for the following reaction is
3.2 10-7.2 H2S(g) 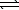 2 H2(g) + S2(g)
2 H2(g) + S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
A) 8.5 10-5 atm
B) 6.2 10-3 atm
C) 9.0 10-3 atm
D) 1.1 10-2 atm
E) 1.4 10-2 atm
Correct Answer:
Verified
Q52: In an experiment,0.42 mol H2 and 0.42
Q53: A sample of solid NH4NO3 was placed
Q54: At 25
Q55: A mixture of nitrogen and hydrogen was
Q56: For the equilibrium N2O4(g) 
Q58: At 700 K,Kp for the following
Q59: For the equilibrium PCl5(g) 
Q60: A 3.00-liter flask initially contains 3.00
Q61: Consider the following equilibrium at 25°C:
2ICl(g)
Q62: At a given temperature,K = 0.021 for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents