Given the following chemical equilibria,
N2(g) + O2(g) 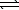 2 NO(g) K1
2 NO(g) K1
N2(g) + 3 H2(g) 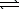 2 NH3(g) K2
2 NH3(g) K2
H2(g) + 1/2 O2(g) 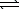 H2O(g) K3
H2O(g) K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g) + 5 O2(g) 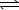 4 NO(g) + 6 H2O(g) K c
4 NO(g) + 6 H2O(g) K c
A) 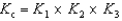
B) 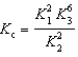
C) 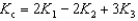
D) 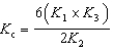
E) 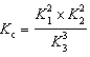
Correct Answer:
Verified
Q64: Assume that the following
Q65: For the reaction N2O4(g) 
Q66: If Kc = 0.152 for A2 +
Q68: The thermochemical equation for the formation
Q68: The symbol Q is called the _.
Q70: A flask contains the following chemical system
Q71: Which of the following equilibria would not
Q72: Consider the following equilibrium:
PCl5(g) 
Q73: Given the following equilibria,
PbBr2(s) 
Q74: Given the following equilibria,
Ni2+(aq)+ 2 OH-(aq)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents