Assume that the following chemical reaction is at equilibrium.
C(s) + H2O(g) 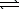 H2(g) + CO(g)
H2(g) + CO(g)
Which of the following statements is/are CORRECT?
1) Increasing the concentration of H2(g) will cause the reaction to proceed in the backward direction,increasing the equilibrium concentration of H2O(g) .
2) Decreasing the temperature will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g) .
3) Increasing the amount of C(s) will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g) .
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
Correct Answer:
Verified
Q59: For the equilibrium PCl5(g) 
Q60: A 3.00-liter flask initially contains 3.00
Q61: Consider the following equilibrium at 25°C:
2ICl(g)
Q62: At a given temperature,K = 0.021 for
Q63: Given the equilibrium constants for the
Q65: For the reaction N2O4(g) 
Q66: If Kc = 0.152 for A2 +
Q68: The symbol Q is called the _.
Q68: The thermochemical equation for the formation
Q69: Given the following chemical equilibria,
N2(g)+ O2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents