The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows.
N2(g) + 3 H2(g) 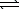 2 NH3(g) H = -92.2 kJ
2 NH3(g) H = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
A) adding N2(g)
B) removing NH3(g)
C) adding a catalyst
D) decreasing the temperature
E) removing H2(g)
Correct Answer:
Verified
Q63: Given the equilibrium constants for the
Q64: Assume that the following
Q65: For the reaction N2O4(g) 
Q66: If Kc = 0.152 for A2 +
Q68: The symbol Q is called the _.
Q69: Given the following chemical equilibria,
N2(g)+ O2(g)
Q70: A flask contains the following chemical system
Q71: Which of the following equilibria would not
Q72: Consider the following equilibrium:
PCl5(g) 
Q73: Given the following equilibria,
PbBr2(s) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents