An SHE electrode has been assigned a standard reduction potential,E ,of 0.00 Volts.Which reaction occurs at this electrode?
A) 2 H2O( 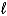 ) + 2 e- H2(g) + 2 OH-(aq)
) + 2 e- H2(g) + 2 OH-(aq)
B) O2(g) + 4 e- 2 O2-(aq)
C) Hg2Cl2(s) + 2 e- 2 Hg( 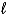 ) + 2 Cl-(aq)
) + 2 Cl-(aq)
D) Li+(aq) + e- Li(s)
E) 2 H+(aq) + 2 e- H2(g)
Correct Answer:
Verified
Q32: The fuel cells used aboard NASA's
Q33: Consider the following half-reactions:
Ag+(aq)+ e-
Q34: Write a balanced chemical equation for
Q35: What is the correct cell notation for
Q36: What is a correct cell notation
Q38: According to the following cell notation,which species
Q39: Given:
2H+(aq)+ 2e-
Q40: The unit for electromotive force,emf,is the Volt.A
Q41: Calculate Q42: Calculate Ecell for the following electrochemical cell![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents