Diethyl ether,used as a solvent for extraction of organic compounds from aqueous solutions,has a high vapor pressure which makes it a potential fire hazard in laboratories in which it is used.How much energy is released when 100.0 g is cooled from 53.0°C to 10.0°C?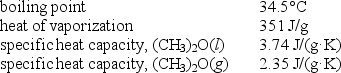
A) 10.1 kJ
B) 13.1 kJ
C) 16.1 kJ
D) 45.2 kJ
E) 48.6 kJ
Correct Answer:
Verified
Q1: Consider the following phase diagram and identify
Q2: Examine the following phase diagram and identify
Q4: Examine the following phase diagram and identify
Q5: Neon condenses due to
A)dipole-dipole forces.
B)London dispersion forces.
C)hydrogen
Q6: Examine the following phase diagram and determine
Q7: Examine the phase diagram for the substance
Q8: Octane has a vapor pressure of 40.torr
Q11: Liquid sodium can be used as
Q18: Pentane, C5H12, boils at 35°C. Which
Q24: In hydrogen iodide_ are the most important
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents