Given the table of specific heat values below,what is the identity of a 10.0 g metal sample that increases by 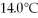
When 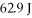
Of energy is absorbed?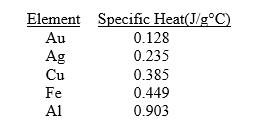
A) Fe
B) Al
C) Au
D) Ag
E) none of the above
Correct Answer:
Verified
Q102: What is the value of 23°C on
Q104: What is the value of -25°C on
Q105: What is the value of 783 K
Q107: How many joules are in 55.2 calories?
A)13,200
B)55,200
C)13.2
D)231
E)none
Q110: Given the table of specific heat values
Q111: How much heat (kJ)is absorbed by 948.0
Q114: How many grams of water when supplied
Q115: How much heat (kJ)is needed to raise
Q116: From the following list of substances and
Q116: What is the final temperature of 25.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents