Refer to the figure below. 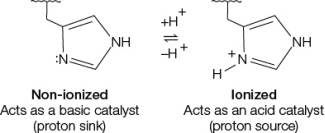 The figure shows how a histidine group at the active site of an enzyme could function either as a base (left) or as an acid (right) .The pKa of histidine is 6.0, which means that at pH 6.0, there are equal numbers of ionized and nonionized histidine groups since a pKa is an acid dissociation constant.With this in mind, what would you expect about the pH sensitivity of enzyme X that uses histidine as a base catalyst compared with enzyme Y that uses histidine as an acid catalyst?
The figure shows how a histidine group at the active site of an enzyme could function either as a base (left) or as an acid (right) .The pKa of histidine is 6.0, which means that at pH 6.0, there are equal numbers of ionized and nonionized histidine groups since a pKa is an acid dissociation constant.With this in mind, what would you expect about the pH sensitivity of enzyme X that uses histidine as a base catalyst compared with enzyme Y that uses histidine as an acid catalyst?
A) Enzyme X and enzyme Y would both have the highest activities at pH 6.0, with lower activities at pH values above and below pH 6.0.
B) Enzyme X and enzyme Y would both have lower activities at pH values below 6.0 and higher activities at pH values above 6.0.
C) Enzyme X and enzyme Y would both have higher activities at pH values below 6.0 and lower activities at pH values above 6.0.
D) Enzyme X would have higher activities below pH 6.0 and lower activities above pH 6.0, while enzyme Y would have lower activities below pH 6.0 and higher activities above pH 6.0.
E) Enzyme X would have lower activities below pH 6.0 and higher activities above pH 6.0, while enzyme Y would have higher activities below pH 6.0 and lower activities above pH 6.0.
Correct Answer:
Verified
Q187: What features of an enzyme's active site
Q188: The activity of an enzyme was examined
Q189: Which is an example of saturation of
Q190: Which part of an enzyme molecule may
Q191: Which statement is not true about enzymes?
A)
Q193: Coenzymes differ from enzymes in that coenzymes
Q194: The formation of covalent bonds between active
Q195: The conversion of glucose to glucose 6-phosphate
Q196: Which of the following can participate in
Q197: Refer to the figure below. 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents