The moment of inertia of a fluorine (F2) molecule is 3.167 × 10-46. What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J ∙ s, 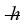 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 4.2 × 10-2 eV
B) 2.1 × 10-3 eV
C) 4.6 × 10-2 eV
D) 5.1 × 10-2 eV
E) 5.6 × 10-2 eV
Correct Answer:
Verified
Q1: A rotating diatomic molecule has rotational quantum
Q6: A certain molecule has 2.00 eV of
Q8: In a p-type semiconductor,a hole is
A) a
Q11: A diatomic molecule has 18 × 10-5
Q13: Ionic bonding is due to
A) the sharing
Q14: A vibrating diatomic molecule has vibrational quantum
Q16: Covalent bonding is due to
A) the sharing
Q17: When a certain diatomic molecule undergoes a
Q19: A diatomic molecule is vibrating in its
Q21: The Fermi energy of rubidium at a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents