Which of the structures below would be trigonal planar (a planar triangle) ? (Electrical charges have been deliberately omitted. ) 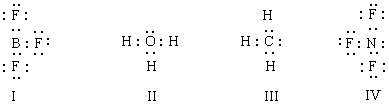
A) I
B) II
C) III
D) IV
E) I and IV
Correct Answer:
Verified
Q84: What is the approximate hybridization state of
Q88: What is the approximate hybridization state of
Q95: What geometry does the methyl cation,CH3+,have?
A) Octahedral
B)
Q96: What is the hybridization of the C
Q99: In which of the following would you
Q100: The bond angle for the C-C-O
Q101: Identify the atomic orbitals in the N-O
Q102: The bond angle for the H-C-O
Q104: What bond angle is associated with
Q126: The bond angle for the C-P-C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents