Identify the atomic orbitals in the N-O sigma bond in the following oxime: 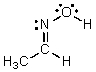
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2sp2,2sp3)
E) (2sp,1s)
Correct Answer:
Verified
Q88: What is the approximate hybridization state of
Q96: What is the hybridization of the C
Q98: Which of the structures below would be
Q99: In which of the following would you
Q100: The bond angle for the C-C-O
Q102: The bond angle for the H-C-O
Q104: The bond angle for the C-N-O
Q105: Which of the following would have a
Q106: What would be the spatial arrangement of
Q126: The bond angle for the C-P-C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents