What is the hybridization of the C atom in the following molecule? 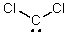
A) s
B) p
C) sp
D) sp2
E) sp3
Correct Answer:
Verified
Q84: What is the approximate hybridization state of
Q88: What is the approximate hybridization state of
Q91: Identify the atomic orbital the lone pair
Q92: VSEPR theory predicts an identical shape for
Q95: What geometry does the methyl cation,CH3+,have?
A) Octahedral
B)
Q98: Which of the structures below would be
Q99: In which of the following would you
Q100: The bond angle for the C-C-O
Q101: Identify the atomic orbitals in the N-O
Q104: What bond angle is associated with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents