Use the following to answer the question: 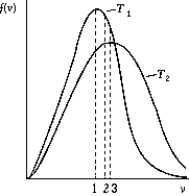
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
A) The curve labeled T1 represents the distribution for the higher temperature molecules.
B) The point labeled "1" corresponds to the rms speed of the molecules whose temperature is T1.
C) The point labeled "2" corresponds to the maximum speed of the molecules whose temperature is T1.
D) The point labeled "3" corresponds to the average speed of the molecules whose temperature is T1.
E) None of these is correct.
Correct Answer:
Verified
Q56: A volume of an ideal gas goes
Q57: An ideal gas whose original temperature and
Q58: Which of the following is NOT an
Q59: At what Kelvin temperature does the
Q60: At what common Celsius temperature is the
Q62: The rms speed of oxygen molecules is
Q63: Use the following to answer the question:
Q64: Five molecules of a gas have the
Q65: A 1 L container contains O2
Q66: A room measures 3 m
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents