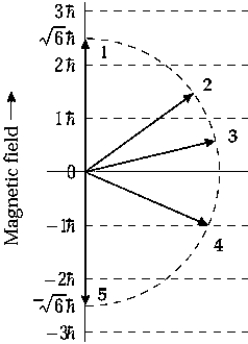
 The orbital angular momentum of an electron in a D state has a magnitude of
The orbital angular momentum of an electron in a D state has a magnitude of 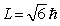 . The figure shows orientations of the angular momentum vector or an electron placed in a magnetic field in the positive z direction. The vector that represents a correct orientation when the field is applied is
. The figure shows orientations of the angular momentum vector or an electron placed in a magnetic field in the positive z direction. The vector that represents a correct orientation when the field is applied is
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:
Verified
Q25: In the Bohr Model of the hydrogen
Q28: What is the magnitude of the change
Q41: The possible orbital quantum numbers of an
Q49: The principle quantum number of an electron
Q53: The symbol that represents the orbital quantum
Q54: In the Bohr Model of the
Q56: The symbol that represents the orbital angular
Q57: If the angular momentum is characterized by
Q58: The symbol that represents the principal quantum
Q59: A hydrogen atom that has an electron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents