The principle quantum number of an electron is 3. The possible magnitudes of the orbital angular momentum, L, are
A) 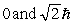
B) 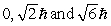
C) 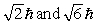
D) 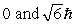
E) None of these is correct.
Correct Answer:
Verified
Q28: What is the magnitude of the change
Q41: The possible orbital quantum numbers of an
Q44: A possible value of the orbital angular
Q46: If the angular momentum is characterized by
Q46: Consider a beryllium (Z = 4)ion with
Q48: If the angular momentum is characterized by
Q50: For the principal quantum number n =
Q53: The symbol that represents the orbital quantum
Q54: In the Bohr Model of the
Q54: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents