On the basis of the kinetic theory of gases, when the absolute temperature is doubled, the average kinetic energy of the gas molecules changes by a factor of
A) 16
B) 2
C) 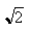
D) 4
E) 0.5
Correct Answer:
Verified
Q31: A cylinder of volume 50 L contains
Q43: Two monoatomic gases,helium and neon,are mixed
Q47: One mole of hydrogen gas (molar
Q53: Which of the following is an assumption
Q55: Doubling the Kelvin temperature of a gas
Q61: Use the following to answer the question:
Q62: The rms speed of oxygen molecules is
Q63: A 1 L container contains O2
Q69: If the rms speed of oxygen molecules
Q78: In a Maxwell-Boltzmann distribution function of molecular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents