Multiple Choice
Two cylinders are connected by a small tube which has a needle valve in the middle. One cylinder contains 4He gas, and the other cylinder contains CO2 gas. The gases are at the same temperature, pressure and volume. The lids that seal the gases in are free to move up or down. When the valve is opened, what happens to the volume in each cylinder? 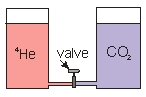
A) The volumes do not change.
B) The CO2 volume decreases and the 4He increases.
C) The CO2 volume increases and the 4He decreases.
D) What happens depends on the initial temperature.
E) What happens depends on the initial pressure.
Correct Answer:
Verified
Related Questions

