As the ideal gas expands from pressure P1 and volume V1 to pressure P2 and volume V2 along the indicated straight line,it is possible that: 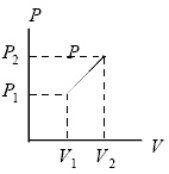
A) the temperature stays constant.
B) the internal energy decreases.
C) the gas is changing state.
D) all of the above are impossible for this particular graph.
Correct Answer:
Verified
Q5: Which of the following increases the internal
Q22: A heat engine exhausts 3 000 J
Q23: A heat engine receives 6 000 J
Q25: A turbine takes in 1 000-K steam
Q28: An ideal gas at pressure,volume,and temperature,P0,V0,and T0,respectively,is
Q29: A thermodynamic process that happens very quickly
Q29: A cylinder containing an ideal gas has
Q30: An ideal gas at pressure,volume,and temperature,P0,V0,and T0,respectively,is
Q31: How much thermal energy must be added
Q32: A heat engine operating between a pair
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents