An ideal gas at pressure,volume,and temperature,P0,V0,and T0,respectively,is heated to point A,allowed to expand to point B also at A's temperature 2T0,and then returned to the original condition.The internal energy decreases by 3P0V0/2 going from point B to point T0.In going around this cycle once,which quantity equals zero? 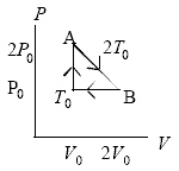
A) the net change in internal energy of the gas
B) the net work done by the gas
C) the net heat added to the gas
D) All three are zero.
Correct Answer:
Verified
Q5: Which of the following increases the internal
Q23: A heat engine receives 6 000 J
Q25: A turbine takes in 1 000-K steam
Q27: As the ideal gas expands from pressure
Q29: A thermodynamic process that happens very quickly
Q29: A cylinder containing an ideal gas has
Q30: An ideal gas at pressure,volume,and temperature,P0,V0,and T0,respectively,is
Q31: How much thermal energy must be added
Q32: A heat engine operating between a pair
Q33: An electrical generating plant operates at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents