Multiple Choice
Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest oxidizing agent. 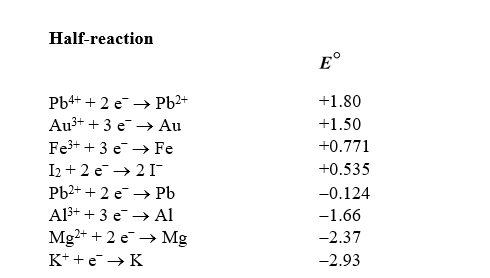
A) Pb4+
B) Pb2+
C) K+
D) K
E) Al
Correct Answer:
Verified
Related Questions
Q54: Using the following standard reduction potentials,calculate the
Q55: Where do you expect to find elements
Q56: Use the table of standard reduction potentials
Q57: Using the following data,determine the standard
Q58: What is the standard cell potential for
Q60: Using the following data,determine 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents