Using the following data,determine the standard cell potential 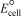 for the electrochemical cell constructed based on the following unbalanced reaction expression: Al(s) + Cr3+(aq) Al3+(g) + Cr2+(aq) .
for the electrochemical cell constructed based on the following unbalanced reaction expression: Al(s) + Cr3+(aq) Al3+(g) + Cr2+(aq) .
Half-reaction Standard reduction potential
Al3+(aq) + 3 e- Al(s) -1.66
Cr3+(aq) + e- Cr2+(aq) -0.41
A) "-1.25 V
B) "+1.25 V"
C) "+2.07 V"
D) "-0.43 V"
E) "+0.43 V"
Correct Answer:
Verified
Q52: Based on the information in the table
Q53: Silver tarnish (Ag2S)can be removed by immersing
Q54: Using the following standard reduction potentials,calculate the
Q55: Where do you expect to find elements
Q56: Use the table of standard reduction potentials
Q58: What is the standard cell potential for
Q59: Use the table of standard reduction potentials
Q60: Using the following data,determine 
Q61: Calculate Q62: An electronic device requires two 1.50-V![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents