Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest reducing agent.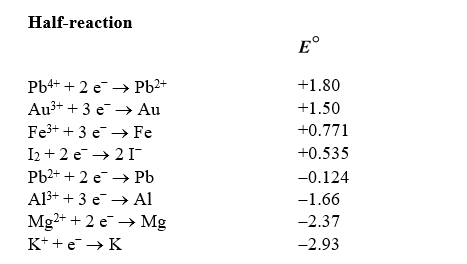
A) Pb4+
B) Pb2+
C) K+
D) K
E) Al
Correct Answer:
Verified
Q51: Using the following data,determine 
Q52: Based on the information in the table
Q53: Silver tarnish (Ag2S)can be removed by immersing
Q54: Using the following standard reduction potentials,calculate the
Q55: Where do you expect to find elements
Q57: Using the following data,determine the standard
Q58: What is the standard cell potential for
Q59: Use the table of standard reduction potentials
Q60: Using the following data,determine 
Q61: Calculate ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents