Using the following data,determine 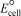 for the electrochemical cell constructed using the reaction of nitrous oxide and oxalic acid under acidic conditions.Unbalanced: N2O(g) + H2C2O4(s) 2 CO2(g) + N2(g) + H2O(
for the electrochemical cell constructed using the reaction of nitrous oxide and oxalic acid under acidic conditions.Unbalanced: N2O(g) + H2C2O4(s) 2 CO2(g) + N2(g) + H2O(  )
)
Half-reaction Standard reduction potential
2 CO2(g) + 2 H+(aq) + 2 e- H2C2O4(s) -0.49
N2O(g) + 2 H+(aq) + 2 e- N2(g) + H2O(  ) +1.78
) +1.78
A) "+1.29 V"
B) "-1.29 V"
C) "+2.27 V"
D) "-2.27 V"
E) "+0.87 V"
Correct Answer:
Verified
Q46: Based on the information in the
Q47: The bromate ion,BrO3-,can form in drinking water
Q48: Using the following standard reduction potentials,calculate the
Q49: Use the table of standard reduction potentials
Q50: Copper is oxidized by nitric acid.If this
Q52: Based on the information in the table
Q53: Silver tarnish (Ag2S)can be removed by immersing
Q54: Using the following standard reduction potentials,calculate the
Q55: Where do you expect to find elements
Q56: Use the table of standard reduction potentials
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents