Using the Following Standard Reduction Potentials,calculate the Cell Potential for the Voltaic
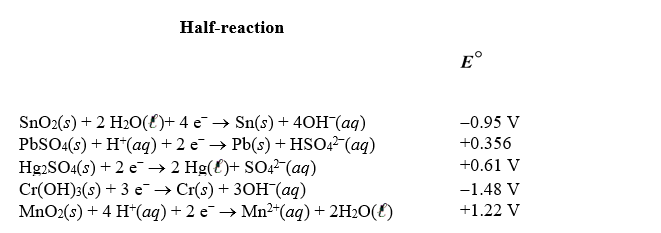
Using the following standard reduction potentials,calculate the cell potential for the voltaic cell that would have the largest 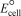 .
. 
A) 2.70 V
B) 2.43 V
C) 2.17 V
D) 2.09 V
E) 1.83 V
Correct Answer:
Verified
Q43: Identify the strongest reducing agent in the
Q44: Based on the information in the table
Q45: Using the following data,determine the standard
Q46: Based on the information in the
Q47: The bromate ion,BrO3-,can form in drinking water
Q49: Use the table of standard reduction potentials
Q50: Copper is oxidized by nitric acid.If this
Q51: Using the following data,determine 
Q52: Based on the information in the table
Q53: Silver tarnish (Ag2S)can be removed by immersing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents