The bromate ion,BrO3-,can form in drinking water if ozone disinfection is performed under basic conditions when bromide ions are present.If this redox reaction were performed in an electrochemical cell,what would the standard cell potential be? The relevant reduction reactions and standard reduction potentials are given below. 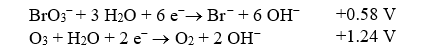
A) "-3.14 V"
B) "+3.14 V"
C) "+1.05 V"
D) "+1.82 V"
E) "+0.66 V"
Correct Answer:
Verified
Q42: Silver tarnish (Ag2S)can be removed by
Q43: Identify the strongest reducing agent in the
Q44: Based on the information in the table
Q45: Using the following data,determine the standard
Q46: Based on the information in the
Q48: Using the following standard reduction potentials,calculate the
Q49: Use the table of standard reduction potentials
Q50: Copper is oxidized by nitric acid.If this
Q51: Using the following data,determine 
Q52: Based on the information in the table
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents