For the chemical equilibrium aA +bB cC,the value of the equilibrium constant K is 10.0.What is the value of the equilibrium constant for the reaction 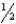 cC
cC 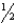 aA +
aA + 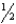 bB?
bB?
A) 0.316
B) 10.0
C) 3.16
D) 0.200
E) 31.6
Correct Answer:
Verified
Q41: In equilibrium expressions, the concentrations of pure
Q43: If the reaction quotient Q has a
Q52: For the equilibrium H2O(g)+ CH4(g)
Q53: Consider the equilibrium A + B
Q55: For the equilibrium 2 NOBr(g)
Q56: For the equilibrium 2 ClO(g)
Q58: An equilibrium that strongly favors products has
Q59: Cadmium and cyanide ions can form
Q60: The following reactions at 1000.0 K
Q74: Addition of reactants to a chemical reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents