For the equilibrium H2O(g) + CH4(g) CO(g) + 3 H2(g) ,Kp = 1.61 10-5 at 1400 K.At a given point,the partial pressures of the gases are 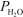 = 0.264 atm,
= 0.264 atm, 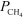 = 0.126 atm,
= 0.126 atm, 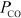 = 0.0382 atm,and
= 0.0382 atm,and 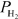 = 0.0974 atm.Which of the following statements is true?
= 0.0974 atm.Which of the following statements is true?
A) Q < K so the reaction will continue to make more products.
B) Q > K so the reaction will consume products to make more reactants.
C) Q = K so the system is at equilibrium.
D) The value of K will decrease until it is equal to Q.
E) The value of K will increase until it is equal to Q.
Correct Answer:
Verified
Q43: If the reaction quotient Q has a
Q47: Write the expression for the reaction
Q48: For the equilibrium 2 HI(g)
Q50: A series of four equilibrium steps
Q51: The equilibrium constants for the two
Q53: Consider the equilibrium A + B
Q55: For the equilibrium 2 NOBr(g)
Q56: For the equilibrium 2 ClO(g)
Q57: For the chemical equilibrium aA +bB
Q60: If the reaction quotient Q has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents