For the equilibrium 2 NOBr(g) 2 NO(g) + Br2(g) ,Kc = 1.56 10-3 at 300.0 K.What is Kp at 300.0 K for the equilibrium,NO(g) + 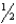 Br2(g) NOBr(g) ?
Br2(g) NOBr(g) ?
A) 5.10
B) 126
C) 1.58 104
D) 0.0384
E) 26.0
Correct Answer:
Verified
Q43: If the reaction quotient Q has a
Q50: A series of four equilibrium steps
Q51: The equilibrium constants for the two
Q52: For the equilibrium H2O(g)+ CH4(g)
Q53: Consider the equilibrium A + B
Q56: For the equilibrium 2 ClO(g)
Q57: For the chemical equilibrium aA +bB
Q58: An equilibrium that strongly favors products has
Q59: Cadmium and cyanide ions can form
Q60: The following reactions at 1000.0 K
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents