The energy profiles for four different reactions are shown.Which reaction requires the most energetic collisions to reach the transition state? 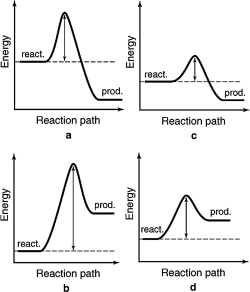
A) a
B) b
C) c
D) d
E) more information is required
Correct Answer:
Verified
Q63: The linear form of the Arrhenius equation
Q79: The linear form of _ is very
Q90: The linear form of the Arrhenius
Q106: Which of the following statements regarding collision
Q107: The following energy profiles for four different
Q109: The following figure shows Arrhenius plots for
Q111: The following figure shows Arrhenius plots for
Q112: The rate constant for the first-order
Q113: The energy profiles for four different reactions
Q114: Which point,as labeled by an asterisk (*)on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents