The energy profiles for four different reactions are shown.Which of the reactions will have the smallest rate constant? 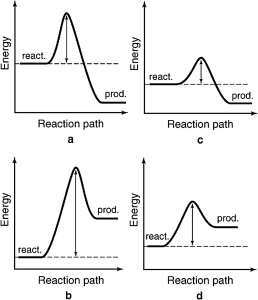
A) a
B) b
C) c
D) d
E) more information is required
Correct Answer:
Verified
Q63: The linear form of the Arrhenius equation
Q79: The linear form of _ is very
Q109: The following figure shows Arrhenius plots for
Q110: The energy profiles for four different reactions
Q111: The following figure shows Arrhenius plots for
Q112: The rate constant for the first-order
Q114: Which point,as labeled by an asterisk (*)on
Q116: The following figure shows Arrhenius plots for
Q117: The following energy profiles for four different
Q118: The following figure shows Arrhenius plots for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents