Consider the reaction: 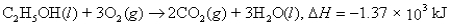 When a 21.1-g sample of ethyl alcohol (molar mass = 46.07 g/mol) is burned,how much energy is released as heat?
When a 21.1-g sample of ethyl alcohol (molar mass = 46.07 g/mol) is burned,how much energy is released as heat?
A) 0.458 kJ
B) 0.627 kJ
C) 6.27 102 kJ
D) 2.89 104 kJ
E) 2.18 kJ
Correct Answer:
Verified
Q53: How much heat is liberated at
Q54: The total volume of hydrogen gas
Q55: At 25°C,the following heats of reaction are
Q56: When 0.236 mol of a weak
Q57: The heat of combustion of benzene,C6H6,is -41.74
Q59: The
Q60: A bomb calorimeter has a heat
Q61: _ involves the transfer of energy between
Q62: Choose the correct equation for the
Q63: Which of the following is both a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents