The total volume of hydrogen gas needed to fill the Hindenburg was 2.11 108 L at 1.00 atm and 24.7°C.How much energy was evolved when it burned? 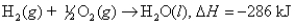
A) 8.64 106 kJ
B) 2.98 1010 kJ
C) 3.02 104 kJ
D) 2.47 109 kJ
E) 4.94 109 kJ
Correct Answer:
Verified
Q49: The change in enthalpy can always be
Q50: If a student performs an endothermic
Q51: Given the equation S(s)+ O2(g)
Q52: Consider the following processes:
2A
Q53: How much heat is liberated at
Q55: At 25°C,the following heats of reaction are
Q56: When 0.236 mol of a weak
Q57: The heat of combustion of benzene,C6H6,is -41.74
Q58: Consider the reaction: Q59: The ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents