Reaction of methane with oxygen really proceeds in two steps: 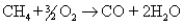
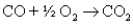 A sample of CH4 is burned in an excess of O2 to give 3.0 moles of H2O.How many moles of CH4 were in the original sample?
A sample of CH4 is burned in an excess of O2 to give 3.0 moles of H2O.How many moles of CH4 were in the original sample?
A) 1.5
B) 2.0
C) 6.0
D) 0.8
E) 3.0
Correct Answer:
Verified
Q86: Suppose the reaction Ca3(PO4)2 + 3H2SO4
Q87: When 233.1 g of ethylene (C2H4)burns in
Q88: Phosphoric acid can be prepared by
Q89: The limiting reactant in a reaction
A)has the
Q90: The refining of aluminum from bauxite
Q92: SO2 reacts with H2S as follows:
Q93: Consider the following reaction: Q94: When rubidium metal is exposed to air,two Q95: Iron is produced from its ore by Q96: What would be the g Al /![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents