Multiple Choice
Iron is produced from its ore by the reactions: 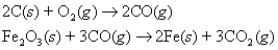 How many moles of O2(g) are needed to produce 4.6 moles of Fe(s) ?
How many moles of O2(g) are needed to produce 4.6 moles of Fe(s) ?
A) 2.3 mole O2
B) 3.5 mole O2
C) 4.6 mole O2
D) 6.9 mole O2
E) 13.8 mole O2
Correct Answer:
Verified
Related Questions
Q90: The refining of aluminum from bauxite
Q91: Reaction of methane with oxygen really proceeds
Q92: SO2 reacts with H2S as follows:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents