Which of the following statements is (are) always true?
I.In order for a process to be spontaneous,the entropy of the universe must increase.
II.A system cannot have both energy disorder and positional disorder.
III. Suniv = 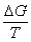 IV.S° is zero for elements in their standard states.
IV.S° is zero for elements in their standard states.
A) I
B) I,IV
C) I,III,IV
D) II,IV
E) II
Correct Answer:
Verified
Q71: Consider the reaction Q72: The standard free energy of formation Q73: Consider the following hypothetical reaction at Q74: Given the following data ( Q75: At 699 K, Q77: When a stable diatomic molecule spontaneously Q78: Which of the following is true Q79: In which process is Q80: Of Q81: Consider the gas phase reaction NO
2N2O5(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents